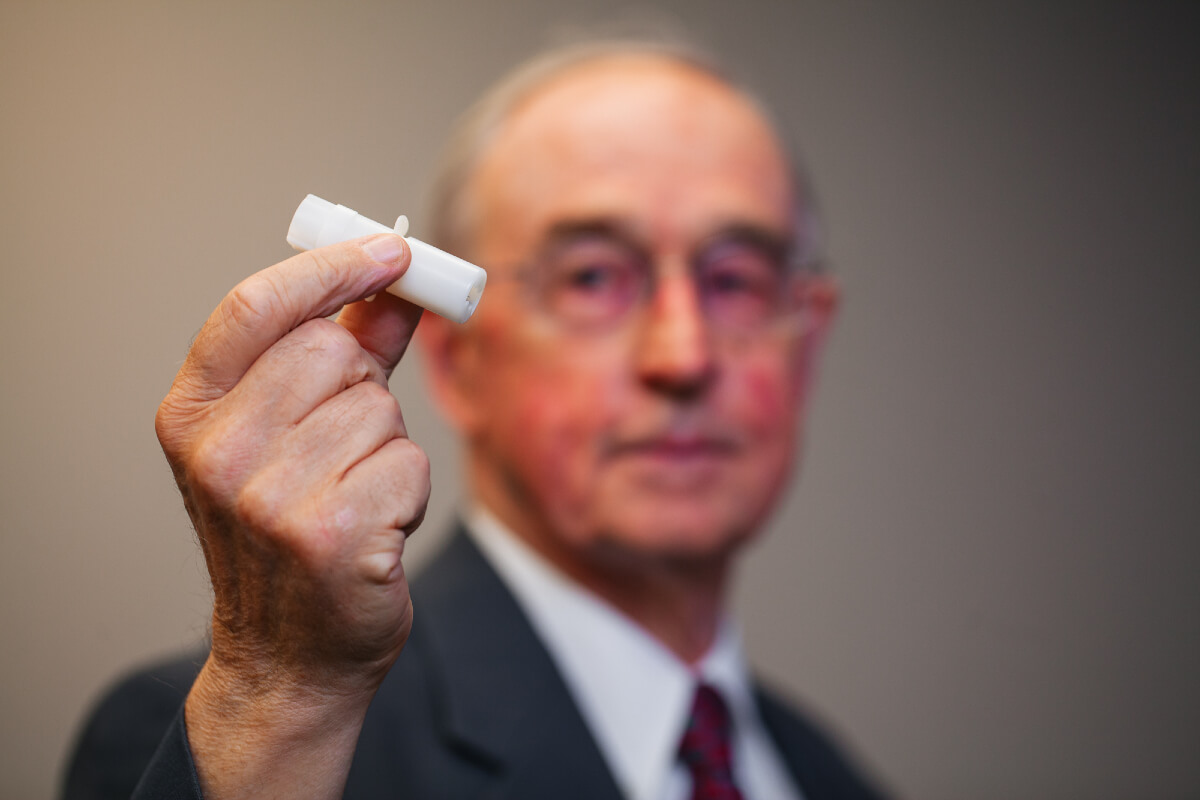
The pandemic has sparked major shifts in the healthcare industry, helping to drive innovation and accelerate the technological revolution. Canadian company PKA SoftTouch is part of this revolution with the impending launch of its highly anticipated pain-free Micro-needle, which is well on its way through the drug approval process after 15 years of research and development. For people with a fear of needles, this will change everything.
New Pain-Free Micro-Needle Will Eliminate Fear of Needles
Although millions of people around the world require regular injections for a variety of health reasons including diabetes and rheumatoid arthritis, many people have a fear of needles, or find them unpleasant and inconvenient. To help people administer medications and vaccines in a painless, cost-effective, and convenient way, Dr. Pankaj Modi, co-founder of PKA SoftTouch, created the pain-free Micro-needle, a patient-friendly delivery system for animals and humans of all ages.
“Twenty-seven percent of the population have needle phobia, and with this device, you eliminate that,” says Dick Crawford, co-founder and CEO of PKA SoftTouch. “People who have to be on a regime with injectable drugs will be more likely to stay on that regime with a painless injection. It’s going to improve quality of care.”
Unlike subcutaneous injections, the device works by injecting medications or vaccines directly into the skin, about two mm deep, targeting the interstitial fluid. The injection is painless as there are no nerve endings in the skin. Other devices are subcutaneous injections below the skin that hit nerve endings causing pain. This makes for a painless process, and it also means people can inject medications like insulin anywhere on the body rather than being limited to the same five points like with a syringe.
Revolutionary Micro-Needle a Boon for Insulin Users
“Right after COVID-19 and vaccines, the next most-used injectable drug is insulin and it’s growing exponentially,” Crawford says. “Diabetes is an out-of-control disease, and there’s no cure. This device is a boon for people who are insulin users.”
Since the device is safe for animals too, both vets and pet owners will find it easier to inject cats and dogs who have diabetes or other medical issues — pets won’t have a fear of needles like with a standard syringe.
Injectable Drug Market Continues to Skyrocket
Projected to reach a value of $1 trillion by 2027, the injectable drug market is an essential component of the pharmaceutical industry. Since the pandemic, the sector has skyrocketed as countries all over the world rush to administer COVID-19 vaccines.
“One of our goals with this device is that we see it being used to inoculate populations in countries where they have worries about Ebola, diphtheria and dengue fever,” Crawford says. “If people see that this device won’t hurt them, they’ll be more likely to go for inoculations.”
Related Articles
Opportunity To Invest in Revolutionary Micro-Needle
The Micro-needle addresses the growing demand for easy administration systems for injectable drugs, in addition to the existing needs of people living with chronic illnesses like diabetes. Not only is it easy to use, but it’s also safely disposable, affordable, and adaptable to a wide range of drugs, medical treatments, and vaccines.
PKA SoftTouch has launched a second round of funding on FrontFundr, Canada’s leading equity crowdfunding platform, to raise money for the device. So far, the company has successfully raised more than $750,000 – 76% of its current campaign goal. Equity crowdfunding allows everyday investors to become co-owners of a company, allowing them to participate in the company’s financial success if they go public. FrontFundr is described as an opportunity for any member of the public to invest in emerging companies that could change the world.
Anyone interested in becoming a shareholder can visit the FrontFundr page to learn about the offering and invest in PKA SoftTouch — investments start from $500 with the online process taking less than 12 minutes.
A Game-Changer in the Injectable Drug Market
In its first round on FrontFundr, PKA SoftTouch welcomed over 300 investors. In 2020, the product won the “Top 10 Best Invention” award in the International Invention Innovation Competition, signifying its status as a game-changer in the injectable drug landscape.
So far, the product has passed all testing with 100% reliability, and is now undergoing animal clinical trials. “The company is at the end of the commercialization cycle, and we’re now in a stage called proof of concept, which involves testing the device against the current gold standard, the syringe,” Crawford says.
Micro-Needle Coming Soon Following Human Trials
“Once we prove our concept works, then we can licence the technology to drug companies to produce the devices and distribute them,” Crawford says. “Why that’s so important to us is that we have 20 or 30 drug companies waiting for these results to begin licensing our technology.”
The next phase of trials will be done on humans at the end of the summer of 2022, an exciting next step in the approvals process. The device is expected to hit the human market in 2023.